What are Humic Substances and How Do They Help with Climate Control by Sequestering Carbon?
-
- Definition of Humic Substances
- Humic Substances Properties
- Humic Structures and Reactions
- How do Humics Remain stable?
- HAs for Soil Enhancement, Water Treatment, Recycling and Carbon Sequestration
- How HA Production from Bio-Waste and Coals helps in Repairing Impaired Lands and Arid Areas while Sequestering Carbon
- How to Calculate Carbon Sequestration Potential from Applications of Humic Substances
- Bibliography
- References
- Additional Reading
How Do Humic Acids Remain Stable?
Chemical or biological mineralization and thermal oxidation of any organic material releases carbon dioxide in the air. If all the carbon behaved in this way we would have only plants (grown by photosynthesis) on the land and carbon dioxide in the air, but there would be no organic matter in the soils. (Some carbon dioxide in the air interacts with inorganic compounds such as limestone on the land and in water sediments, and this helps to somewhat reduce the amount of CO2 in the air). Plants need water, air and soil to grow and reproduce. The fact is that soils exist because humic substances are stable compared to carbohydrates and other plant and animal molecules. Carbon dating indicates that some HSs are 10,000 to 30,000 years old.
We noted above that HSs have functional groups that they use to bind metals and retain water. Their other structural feature is carbon-rich units (“greasy blobs”) that give the HA molecules some hydrophobic (water-repellant) character. One HA model has the hydrophilic (water-loving) functional groups on the outside and the hydrophobic groups on the inside of a molecule that looks like a telephone cord. The molecule presumably can “turn itself inside out” as necessary, making the outside hydrophobic.
The scanning electron micrograph below shows a sample of a solid HA and the Figure shows a proposed molecular structure that has a mainly hydrophilic exterior.
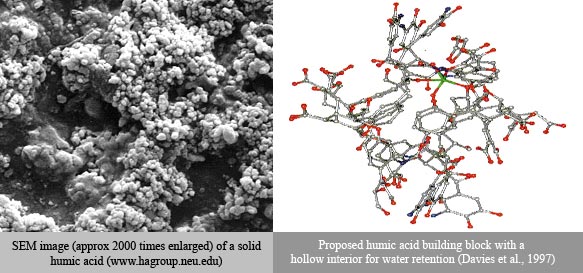
Another way for HAs to resist mineralization is by attaching themselves to minerals using their functional groups. This makes the surface of the mineral “greasy” and leaves the HAs less susceptible to oxidation. A third possibility is that solid HAs have pores that are too small for microbes to pass through, hiding the carbon the microbes need for food.
Research shows that biomass composting and humification proceed in stages of increasing time span. Depending on the biomass source, moisture content, the microenvironment and other factors, the humification stages in the first 60-70 days are exothermic and may result in up to 50% carbon mineralization of the most active components, as monitored by the atomic C/N ratio, which is a measure of humus stability. During humification, the ratio NO3-/NH4+ increases to about 4 and is an index of humified compost maturity.1
A study of municipal solid waste (MSW) composting showed that the total quantity of humic acid (HA) was steady during the several stages of humification and maturation that involve mineralization of oxidatively unstable MSW components.1 This is significant since
- HA is the major humic component of every productive soil [the MSW sample1 was already partly composted]
- HA is the most chemically stable organic soil component
Studies based on radiochemical 14C dating have shown that HAs in Andosols, Chernozems and other soils were formed several thousand years ago and survive to this day.2-15 Attachment to minerals stabilizes HAs11,13 and it is significant that coal-derived HAs have a high mineral content (a typical ash content of lignite-derived HA is 34%w/w)
34%w/w)16.
This is significant because HAs from ancient coal sources are not only stable but also being producted as highly stable cross-linked humic material for water clean up.
The resultant HAs are dispersed in and on soil to improve its ability to:
- Retain water
- Exist as hydrogels [converted by gel processing to give aerogels]
- Buffer soil pH
- Enhance soil structure and texture
- Bind fertilizer and inhibit soil runoff
- Sequester heavy metals
- Catalyze redox reactions
- Sequester and transform PAHs and PCBs in contaminated soils
- Bind RNA and DNA (life perhaps began on surfaces)
- Simultaneously remove TCE, PCE, other POCs and metals from contaminated water at civil, industrial and military facilities
- Prevent beach and soil erosion by binding to minerals, and enable creation of new soil from the desert